About Event
Thank you to our speakers, sponsors, and delegates who joined us in Boston for the summit! If you are interested in the 2025 event, please get in touch at:
The only event helping to improve efficacy of traditional payloads, diversify mechanisms of action in toolkits of payloads and broader therapeutics applications of ADCs in cancer and beyond!
We are currently riding a wave of innovation following major successes from the lower potency Topo1 payloads used in Trodlevy and Enhertu. Drug developers are looking to expand their toolkit of payloads with optimized topo1 inhibitors, tubulin inhibitors and DNA damaging agents, and leveraging completely novel mechanisms of action, such as the NMT inhibitors, amanitin based toxins and oligonucleotide payloads.
This was the opportunity to hear in-depth presentations from leading ADC developers focussed on physiochemical structural modifications and novel discoveries in the ADC payload field.
What You Missed:
Myricx Bio, winner of the Best ADC Poster 2023 at the 10th World ADC Awards, shared validation of their novel N-Myristoyltransferase Inhibitor with pre-clinical efficacy studies
Bolt Biotherapeutics presented their chemistry considerations when developing immune stimulating antibody conjugates with a sneak peek at their proprietary STING-agonist ISAC demonstrating low activity in cytokine release syndrome safety models
Sutro Biopharma, developers of ADCs with TOPO1 inhibitor payloads, showcased their work in analyzing relationships between payload structure alterations and biological activity to provide insights into the molecular determinants of efficacy and toxicity
Merck shared details about their PROxAb Shuttle Platform that enables plug-and-play solutions for targeted PROTAC delivery
YOUR PEERS JOINED TO:
Build upon successes from Topoisomerase payloads and explore novel camptothecin analogues to develop the next-generation of Dxd and Exatecans with Sutro Biopharma and the NIH.
Explore how novel PROTAC degrader payloads bound to antibodies can improve safety and efficacy profiles, how their catalytic nature enables cellular processes to be disrupted, and the role of SAR studies play in enabling antibody conjugation alongside Merck KGaA and Orum Therapeutics.
Learn the impact of different payload types have on your choice of linker and conjugation technology to enable optimal DAR, payload stability and release with Tubulis and Takeda.
Match payload classes with relevant efficacy and toxicities to uncover emerging trends in payload development and assess gaps in payload design during our Exclusive Workshop Sessions.
Explore novel payloads including NMT, RNA Polymerase II inhibitors and KIF20A Kinesin to understand how novel MoA can offer efficacy and safety improvement over payload classes currently in development.
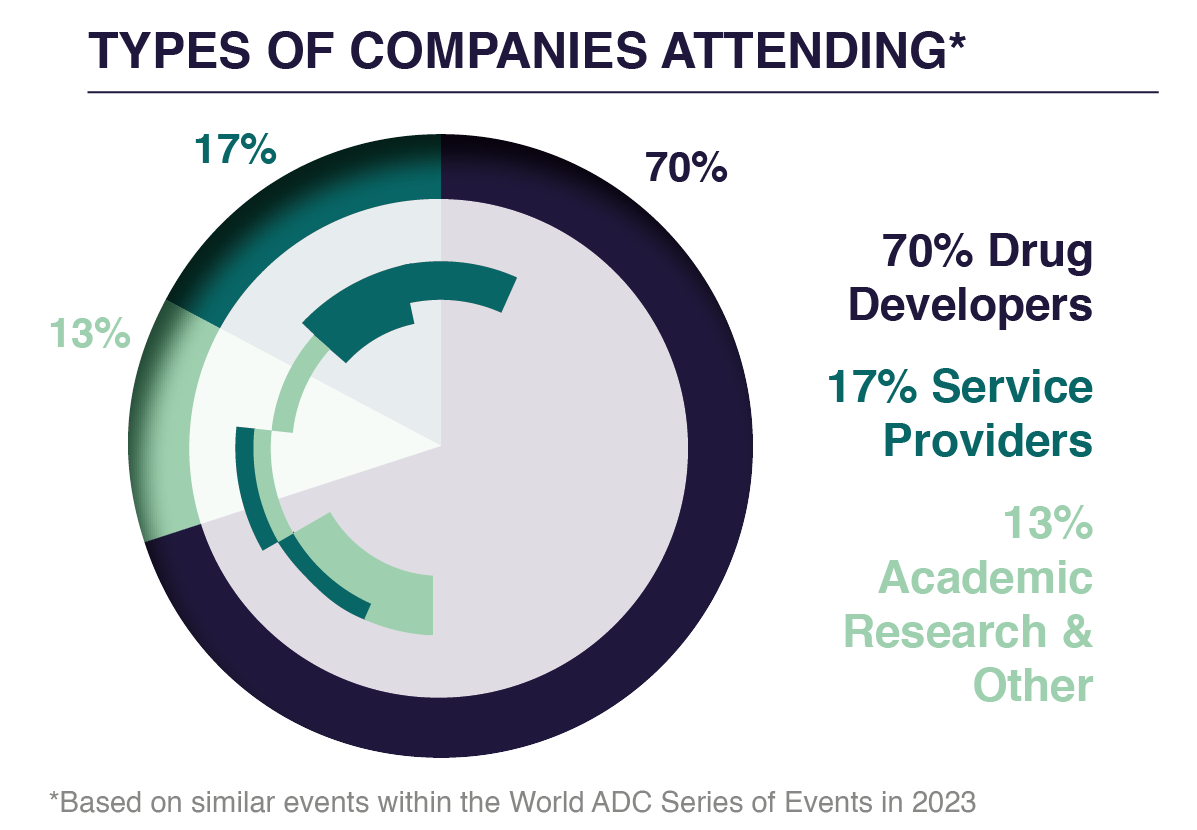
WHO WAS THERE?
WHAT OUR SPEAKERS HAVE TO SAY:
"I am looking forward to seeing the cutting-edge innovation in payloads that will take ADCs to the next level!"
- Romas Kudirka, Director, Chemistry & Bioconjugation, Bolt Biotherapeutics
"I believe it is critical to understand the cellular interactions of the payloads to select potential responders and optimize drug combinations"
- Yves Pommier, Chief, Developmental Therapeutics Branch & Laboratory of Molecular Pharmacology, Center for Cancer Research, National Cancer Institute, NIH

